I was introduced to the principle of Piezoelectricity after watching a video describing how to build a simple contact microphone.
( you can watch it here: DIY Contact Mic – Collin’s Lab (youtube.com) )
Originally invented by ###
When a molecular structure lacks a center of symmetry and gets compressed, the uneven arrangement of atoms causes a charge separation. This results in a net positive charge at one end of the molecule and a net negative charge at the other end. Compression intensifies this effect by altering the spatial arrangement of the atoms.
Rochelle Salt is an easy and safe chemical to make. In it’s crystal is a piezoelectric material.
Ingredients required:
- 1 cup Distilled water (or Deionized [DI] water).
- 80g Cream of Tartar.
- 2 Tablespoons of Sodium Carbonate (Can make by heating baking soda (Sodium Bicarbonate).
- Coffee filter (or equivalent)
- Optional: Activated charcoal
Full reaction:
KHC4H6O6+NaHCO3→KNaC4H6O6+H2O+CO2 –crystallization–→ KNaC4H6O6·4H2O

1. Take 1 Cup Distilled or DI water in a Pyrex measuring cup

2. Place the water filled measuring cup in a pot filled with simmering tap water.

3. Mix 80g Cream of Tartar into the water solution
KHC4H6O6

4. Add a tablespoon of Sodium Carbonate. Prepare for mixture to bubble by producing CO2. After bubbling is finished, add another spoonful, wait for bubbling to subside, repeat until reaction is finished. Solution should become translucent with a yellow hue.
KHC4H6O6+NaHCO3→KNaC4H6O6+H2O+CO2


KNaC4H6O6+H2O

5. Clear solution will have some unreacted Sodium Carbonate that will need to be filtered out. Pour the solution into coffee filter or equivalent (optional to have a layer of activated charcoal to make solution more transparent/remove yellow color).

6. Pour filtered solution into a clean plastic or glass evaporation dish. I used a wide plastic Tupperware container. I placed the lid on loosely to slow evaporation a bit to give the crystals more time to form uniformly.

7. Place the container in a mildly cool place overnight (or until you see satisfactory crystal growth). The water will evaporate from the supersaturated solution and the salt will collect together in a lattice structure KNaC4H4O6·4H2O
KNaC4H6O6+H2O

* The results I got the next day.
KNaC4H6O6·4H2O + H2O

* The small crystals were saved. They can be used as seeds for growing larger crystals in the future if I feel like it.
KNaC4H6O6·4H2O

* This one looked good for piezoelectric testing.
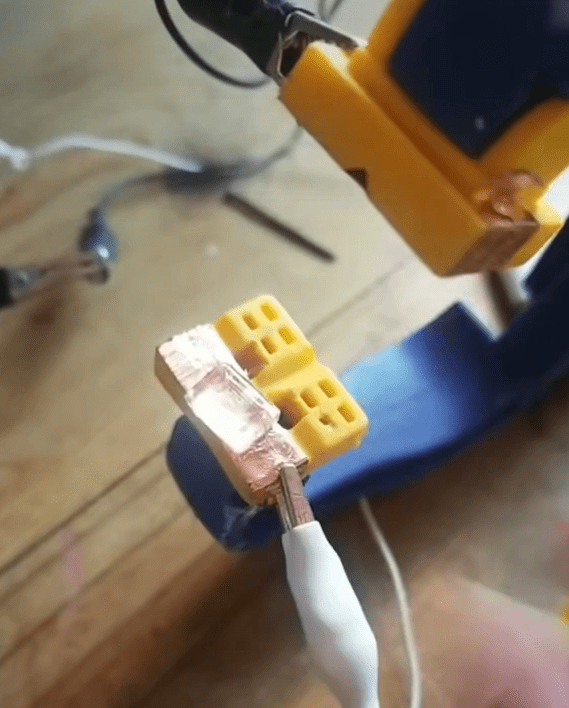
Using a plastic locking clamp with copper tape on each jaw, I clamped electrical contact to the top and bottom of the salt crystal.

I used alligator clips to connect the top and bottom of the crystal to my oscilloscope probe.

Here’s an example signal from tapping the clamp against my desk. The negative 2V peak appears as the crystal would compress from the blunt force and the positive 1.5V peak appears as the crystal decompressed due to elasticity.
The opposite reaction will occur as supplying a potential across the crystal will cause it to mechanically deform. Connecting the crystal to any diaphragm with an audio frequency signal will make a speaker/buzzer.
Here is a diagram of The Rochelle-Salt Crystal Reproducer from the July 1932 Radio-Craft magazine (page 15):

https://onlinebooks.library.upenn.edu/webbin/serial?id=radiocraft
History
Rochelle Salt was discovered by
While looking for a new purgative. Elie mixed wine tartar (cream of tartar) with baking soda. It was a family secret recipe for some time.
The Piezoelectric effect was first written about in 1880 by the brothers
Jacques and Pierre Curie
in the paper
Compressional development of polar electricity in hemihedral crystals with inclined faces (I translated the title but the original paper is in French)


Leave a comment